Female Slings
Description of Procedure
General Information
The female mid-urethral sling is a minimally invasive, outpatient procedure to treat stress urinary incontinence. This surgery has been performed sing the mid 1990's and has been shown to be safe and effective. The sling lies under the urethra acting as a “hammock” which maintains urethral support and keeps it closed during abdominal pressure increases (as with a cough, sneeze, or athletic activity), preventing loss of urine.
The Procedure
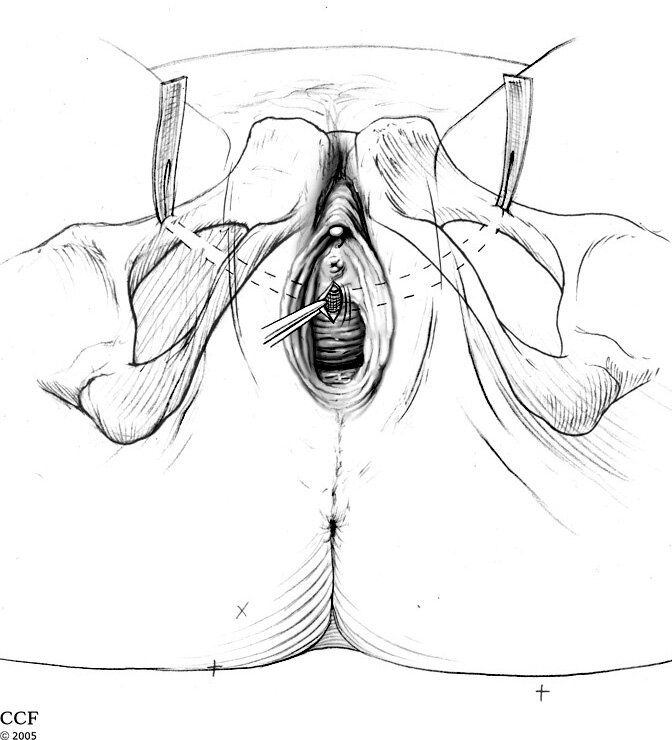
The female sling is a short (15-30 minute) outpatient surgery done under general anesthesia, spinal anesthesia or under sedation with local anesthesia. A 2-3 cm (1 inch) incision is made in the vagina as well as 2 mini incisions just above the pubic bone or in the inner thigh just beside the labia. In some cases only a single vaginal incision is made. The sling, made of surgical mesh (polypropylene), is then placed under the urethra and brought out through the mini incisions using specially designed instruments. The sling is then adjusted to the proper tension (no pressure on the urethra) and then buried under the skin incision after excess mesh is removed. The incisions are closed with dissolvable sutures. Patents usually go home within a few hours after surgery.
The Goals of Surgery
The primary aim is to improve the severity of stress urinary incontinence to a satisfactory level. Most patients (90% or more) will achieve this goal. Some of these patients (about 70-80%) are completely dry. Please note that this surgery is not designed to treat overactive bladder or urge incontinence (see Incontinence Overview for more information about this). In some cases of mixed incontinence, one may experience an improvement in both stress and urge-related leakage events - usually these patients have a unique condition where the urge incontinence is triggered by stress incontinence episodes.
In long-term studies looking at patient-reported outcomes, the majority of properly selected patients have marked improvement in leakage. What can patients expect?
9 out of 10 patients will be dry in the first year after surgery
7 out of 10 patients remain dry at 5 years after the surgery - in most of these the results will last well over 10 years (that is, in many patients the incontinence never comes back)
patients who are elderly or over weight are more likely to have recurrent leakage over time
Potential Risks
In general, the rate of any serious complication is very low (less than 2% overall) making the risk-benefit profile of this surgery highly favourable. Potential risks include:
Bleeding – The chance of significant bleeding requiring hospitalization or blood transfusion is very low (less than 1 in 100). After the procedure, you may experience bleeding similar to a light period for a day or two and may want to use a pad to protect your clothing. You should not have heavy vaginal bleeding or blood in the urine.
Infection – This risk is low and you will be given preventative antibiotics following the procedure
Sling exposure in the vagina (vaginal erosion) – Rarely, the vaginal skin may not heal well and the underlying sling material may be exposed. This usually resolves with time and use of estrogen-type creams over the area. Rarely, surgery is needed to remove the exposed sling.
Injury to the bladder or urethra (including ureethral erosion) – Cystoscopy is performed at the time of the procedure to visualize the bladder and urethra. If bladder perforation occurs, it can almost always be corrected during the procedure and a catheter needs to remain in for about 7 days after surgery to let the bladder heal completely.
Hip and leg pain – This may occur with slings placed through the inner thigh technique. The discomfort usually resolves over several days. This complication is primarily with the TOT procedures - younger and active females who are considering surgery should lean towards TVT.
Urinary Dysfunction Following TVT or other Mid-urethral Slings
The goal of surgery is to correct incontinence - and this is achievied in most though not all patients. Them TOMUS trial found that TVT 81% vvs TOT 78% corrected incontinence.
There are two types of voiding dyfunction that may result from surgery or may be exacerbated by surgery.
Urinary retention – Almost all patients will go home after the surgery without a catheter. It is not uncommon to feel some mild slowing in urination for a few days after surgery. If you can’t void easily you may go home with a catheter for one or two days. In rare cases (less than 2%) a second procedure to loosen or cut the sling may be necessary.
Why: multiple potential reasons; kinking of urethra, hypersuspension (too tight), scaring
Mid-urethral slings thought to have a lower incidence compared to other approaches
TOMUS trial the risk of retention was 3% in TVT group vs. 0% in TOT
How is urinary retention managed? This depends on the circumstances. Fortunately, the risk of needing more surgery is rare - a study reviewed the revision rates in 188,000 women with MUS: 1.3% for revision/removal for retention vs. 2.5% for mesh erosionIn most cases, a wait time of a few weeks shoudl be given to let any edema to settle since permanent retention is rare. However, retention persisting for more than a month should usually be addressed with incision of the sling. Fortunately, most patients will remain continenct - though about 1 in 5 patients will have return of incontinence.
Urinary urgency/urge incontinence – A small number of women may have new onset urinary urgency following the sling procedure. This resolves in most cases, but in some medication or other therapies may be required.
Higher risks with younger patients , concomitant anterior/apical prolapse had higher rates of requiring sling revision/removal
If patients had pre-existing urgency type symptoms were more likely to have worsening post-operative symptoms
How is post-operative urinary urgency managed? This is challenging, especially since many patients will have pre-existing urgency. It is important to ensure that there is no infection or retention - these should be addressed first. The management is otherwise similar to that for overactive bladder.
Canadian Urological Association Position Statement on the Use of Transvaginal Mesh 2016
You can download the position statement here. It is very similar to the one issued by the FDA in 2011.
Food and Drug Administration 2011 Transvaginal Mesh Safety Update
Many patients have read or heard about a notification from the FDA in 2008 (updated in 2011) regarding the potentially serious complications associated with transvaginal synthetic mesh to treat pelvic organ prolapse and stress urinary incontinence. This was accompanied by recommendations on how to lessen risks and counsel pateints. The notice was predominantly directed to mesh for the use of pelvic organ prolapse and not for mid-urethral slings (such as TVT and TOT). We do NOT use any of the 'transvaginal mesh kits' for pelvic organ prolapse for which the notification was primarily intended.
The FDA recently re-analysed the data and the risk of mesh related complications is lower with incontinence type procedures than for pelvic floor mesh. The risks of erosion is in the range of 1-3%.
The American Urogynecologic Society has issued a position statement:
complete restriction on the use of surgical mesh was not the stated intent of the FDA safety communication
the decision on surgical alternatives should be made by the patient and her surgeon
in some circumstances, pelvic organ prolapse may be the most appropriate surgical option
any restriction of mesh slings for the treatment of stress incontinence is clearly not supported by any professional organization or the FDA
any restriction of mesh placed abdominally (as opposed to transvaginally) for the treatment of prolapse is clearly not supported by any professional organization or the FDA
Mesh for stress incontinence has been extensively used for over 15 years. When used in properly selected patients usually results in excellent outcomes. As always, there are risks with surgery including TVT and TOT.
After Procedure
If you have questions that are not answered here, please contact us.
What to Expect
You will begin a voiding trial immediately following surgery. You should void every 2-3 hours. If unable to void you should perform self-catheterization if you have been taught how. Keep track of voided and/or catheterized volumes. If you have not been taught self-catheterization and cannot void you will need to go to your nearest hospital emergency room for a catheter to be placed.
If you have an indwelling catheter please see catheter care instructions.
Urinary frequency, urgency and a slower urine stream may occur in the first 1-2 weeks.
Vaginal bleeding similar to a light period is expected for 1-2 weeks.
May experience pain or discomfort around incision sites.
You may experience inner thigh discomfort.
Cautions
Report any of the following to your doctor:
Heavy vaginal bleeding or clot passage
Catheter blockage
Bright red blood or clots in the urine
Fever over 38.5 C
Severe pain unrelieved by medication
Diet
Clear liquid diet immediately after surgery
Advance to usual diet as tolerated
Activity
Get up and about as soon as possible after surgery
Walk as tolerated
Avoid lifting more than 30 lbs or vigorous physical activity for one month
No intercourse or tampons for 6 weeks
You may start showering 24-36 hrs after surgery. Avoid water stream directly on
incision. You may sponge bathe. Do not submerge in a tub bath for 3-4 weeks.You may start driving about 5-10 days after surgery. Do no drive if you are still on
narcotic pain medication or have limited mobility.Your return to work will be dependent on your job requirements
Medications
Take antibiotics as per prescription
Use prescription pain medication as needed
Take a stool softener (obtain over the counter) starting the night of your surgery. Stop
taking stool softeners once having soft bowel movements. Do not take stool softeners if
diarrhea occurs.If you have not had a bowel movement by the 3rd day after your surgery, take a laxative
(obtain at your local pharmacy over the counter)You may begin your regular medications when you leave the hospital unless instructed
otherwiseIf you take bloodthinners (ASA, plavix, warfarin), your doctor will advise when you can
start them again
On the Web
Urethral Sling Patient Information from Bard (makers of the Align systems)