Infertility and Genetic Conditions
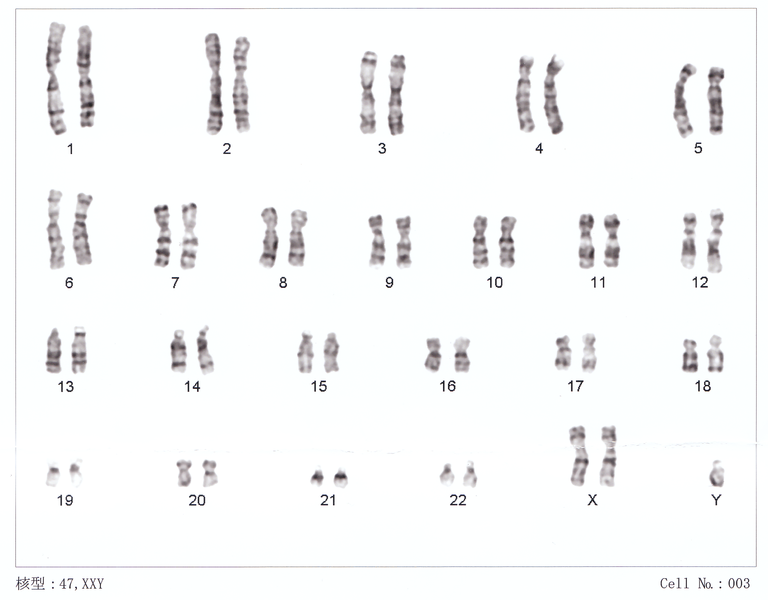
Picture: This a karyotype - an analysis of the complete chromosomal complement.
Genetic abnormalities are increasingly recognized as a cause of infertility. While most men with few or no sperm do not have an identifiable genetic abnormality, a substantial number of men (up to about 15%) will have a genetic abnormality. Many men question the utility of genetic testing since it may take months for the results and because there may be additional costs involved. This is not the right way to think about genetic testing.
Genetic infertility has significant implications. Most importantly, genetic abnormalities carry significant implications related to non-reproductive health of the men who have an abnormality. In men without sperm in the ejaculate (non-obstructive azoospermia), the result can inform us as to the chances that sperm might be present in the testis and the utility of attempting surgical sperm retrieval. Lastly, if sperm are present there may be health implications to a man’s offspring. Without doing the appropriate testing, we will not be able to counsel you about your own health.
The results from genetic testing may take several months (especially the karyotype). In our experience, some men are reluctant to proceed with testing, either because of cost, fear of the stigma of a diagnosis of a genetic abnormality or simply because of indifference. We’re happy to discuss any concerns you may have.
Please note that Canadian law forbids discrimination on the basis of genetic abnormalities and insurance companies may not demand test results. Understand your rights before submitting results.
Background on Genetics & Infertility
-
Chromosomes are microscopic structures which contain the genetic material used to construct and maintain the body. They can be though of as a combination of blueprints and instruction manual. Genes contain basic instructions which direct the cells in the body. Just as missing parts of a blueprint or instructions for a house might mean that the kitchen does not get build or the dishwasher does not work properly, genetic conditions can result in malfunction of the body. A wide variety of genetic abnormalities can result in problems with fertility.
Humans normally have 46 chromosomes. There are 22 types of somatic chromosome of which 2 copies exist (for a total of 44) and 1 pair of sex chromosomes (for a total of 2). The sex chromosomes in males are an X chromosome and a Y chromosome (XY), whereas females have 2 X chromosomes (XX). Males are genetically described as 46 XY and females as 46 XX.
-
Cause of infertility can be categorized several ways
Genetic vs. Non-genetic
Reversible vs. Irreversible
Treatable or non-treatable
Whlle genetic conditions are not 'reversible' with current technology, identifying a genetic condition is critical in infertile males because:
A genetic cause may be transmitted to offspring.
Identifying the specific genetic cause can provide important information on the chances that sperm production is present in the testis even if there are no sperm in the ejaculate.
Some genetic diseases are associated with health problems in addition to infertility. Preventing those problems is important.
-
Genetic abnormalities are very uncommon in men with sperm concentrations >5 million/ml - in fact, the chances of a genetic abnormality if the sperm count is > 5 million/ml is the same as in the general population.
Testing for genetic abnormalities is useful only if there are < 5 million sperm per ml.
-
While there may be clinical features that suggest one cause or another, a precise diagnosis requires genetic testing. All genetic tests are done with a blood sample. A sperm is a complex DNA transport vehicle and virtually all of the chromosomes are involved in sperm production. Note that there are many genetic causes that are currently unknown and that cannot be tested for at this time. The frequently used genetic tests used in infertile men are:
Karyotype: determines the number and type of chromosomes. Large defects in chromosoms can sometimes be tested, but specific genes are not identified. Analogous to checking that all of the volumes of an encyclopedia are present and that the order of the books is correct.
Y-chromosome microdeletion: checks the distal arm of the Y-chromosome which is important in sperm production. See more below
Cystic fibrosis testing: checks for the absence or presence of genes commonly involved in cystic fibrosis and absence of the vas deferens.
It is important to recognize that virtually every genetic abnormality will have a spectrum of severity in terms of clinical presentation. Quite frequently, men who are diagnosed later in life as a result of infertility fall into the milder range of the disease.
-
Despite the large number of genetic abnormalities associated with infertility, there are 4 major types of genetic problems which constitute the bulk of diagnoses.
Klinefelter's Syndrome
Y-chromosome micro-deletions
Congenital Absence of the Vas Deferens (CAVD) and Cystic Fibrosis
Other: aneuploidy, translocations, inversions, etc.
| Infertile Men | ||||
|---|---|---|---|---|
| Gen Pop | All | < 5 million/ml | No Sperm | |
| Overall | 0.5% | 6% | 5% | 15% |
| 47XXY(KS) | 0.2% | 1% | 7-14% | |
| Y-microdeletion | 0.02% | 7% | 3-13% | |
| 47XYY | 0.1% | 1% | 1% | |
| CBAVD | 0.1% | 1% | ||
Table: Probability of a Genetic Abnormality Based Test Population
With increasing genetic testing, Y-chromosome microdeletion is increasingly recognized as the most common identifiable cause of non-obstructive azospermia.
Klinefelter's Syndrome
-
Klinefelter's syndrome (KS) results from the presence of an extra X chromosome in a male. The karyotype in KS is 47 XXY. Under normal circumstances during sperm or egg production, the 46 chromosomes of an individual are divided into two groups of 23 chromosomes. Each egg or sperm contains 23 chromosomes. Once the egg and sperm combine to form an embryo, there are once again 46 total chromosomes, with half coming from the father and half from the mother.
The sex chromosomes (the X and Y chromosomes) normally divide before an egg or sperm is made such that the woman will contribute one of her two sex chromosomes and the man will contribute one as well. Because a woman has 2 X chromosomes, she can only contribute an X to the egg; because males have and X and a Y chromosome, men can contribute either an X or a Y to the sperm. Occasionally, during division of the sex chromosomes either the XX or XY pair will fail to separate (non-disjunction) and the male or female will contribute 2 sex chromosomes instead of 1 (i.e. the extra X chromosome may come from the sperm or the egg). The end result is that the offspring will have an extra sex chromosome - 3 instead of the normal 2; one result of having an extra sex chromosome is the 47 XXY karyotype.
Klinefelter's syndrome is much more common that was previously appreciated. KS affects about 1 in 500 to 1 in 1000 men. In the past, only the most severely affected individuals were identified since they often presented with manifestations of KS other than just infertility. Symptoms may be subtle and many individuals may never be diagnosed. Today, very mild forms of KS can be detected with genetic testing, which is often done for infertility, and would not have been recognized otherwise because the individuals appear perfectly normal. It is important to recognize this because most men with Klinefelter's will not have the types of problems described in the historical literature - such as mental retardation, low testosterone or poor virilization. The fallicies regarding the clinical symptoms of KS may be propegated on the internet. In reality, there is a wide spectrum of clinical features in Klinefelter's syndrome and most men with KS live perfectly normal lives - with the excpetion of reproduction. Many men with KS will have normal intelligence, advanced degrees, appear completely normal and not have any of the behavioural problems which are sometimes mentioned in text books. Some men may have differences in the way they learn and process information, leading to delays in speech and language development. Be cautious when reading about KS on the internet because the information may be outdated.
-
Diagnosis of Klinefelter's disease is done by performing a karyotype on a blood sample. Common clinical features that are suggestive of Klinefelter’s syndrome include small testes, a specific pear-shaped body habitus and slight breast enlargement (gynecomastia). Hormonal testing will usually demonstrate a high follicle stimulating and leutenizing hormone levels plus low testosterone. A low testosterone is not seen in all men with KS, and some may have 'higher than normal' levels of testosterone.
-
Microdissection of the testis (microTESE) can find sperm in about 30-50% of men with Klinefelter's syndrome. Any sperm harvested in this manner require IVF+ICSI for fertilization. Genetic counseling is required in all men prior to consideration of IVF because there is a small risk of transmission to the offspring. Based on the limited success of the frozen/thawed approach, the use of fresh sperm with a coordinated IVF-ICSI cycle is recommended.
There are additional issues pertaining to men with Klinefelter's syndrome including an increased risk of breast cancer, osteoporosis and other rare tumors. Men often require life long testosterone supplementation.
-
The health issues with Klinefelter’s syndrome extend beyond infertility.
Helpful resources can be found on the American Association for Klinefelter Syndrome Information and Support website.
Hormones
Puberty and secondary sexual characteristics (e.g. beard) may be delayed.
Increased breast tissue is common - 50-75% of males will have this.
Testosterone supplementation is frequently utilized, but reproductive goals need to be clarified before administration. When testosterone supplementation is appropriate, it can have beneficial effects on body habits, mood, and behaviour, and decreasing the risk for osteoporosis. An endocrinologist will often initiate and monitor treatment.
Reduced thyroid function (hypothyroidism) is uncommon, but can occur. Thyroid inflammation is common.
Thyroid stimilating hormone and T4 levels should be measured yearly.
Tumors
In comparison to XY men, men with Klinefelter's are at increased risk for germ cell tumors (<1%) and breast cancer (3%).
Germ cell tumors: those tumors involving cells capable of developing into any tissue - such as those found in the testis, but also the retroperitoneum or mediastinum. Very uncommon and there are no standard recommendation for screening tests.
Breast cancer: the risk is markedly elevated compared to other men (20-50x), but the overall incidence is still lower than for women.
Periodic self exam and yearly breast exam by a doctor should be undertaken.
Autoimmune Diseases
Diabetes is more common as are some autoimmune disorders. The exact amount of increased risk is unclear.
Yearly fasting glucose is recommended.
Y-Chromosome Microdeletions
-
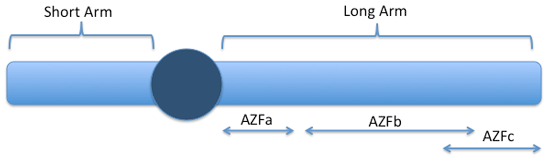
The Y-chromosome contains critical instructions for the production of sperm. If segments of the Y chromosome are missing, then sperm production is usually impaired. The size/length of the deletion and its location are important in determining if sperm production could be present in the testis, even if there are none in the ejaculate. Short deletions limited to the distal end (towards the tip) of the long arm of the Y-chromosome are associated with a very high probability of spermatogenesis being present. Conversely, extensive or proximal defects (towards the center) are usually associated with the complete absence of sperm production. Determing the location and length of the deletion are very important in determining the utility of looking for sperm in the testis.
Note that male offspring will receive the defective Y-chromosome and be affected by infertility as adults. Some males with YMD have sperm production early in life which is lost later on. Therefore, if a couple chooses to use sperm with YMD and has a male offspring, it may be worthwhile to have their son's semen analysis checked early in puberty and to cryopreserve sperm if any are found. Options to avoid transmission include primplantation genetic diagnosis and the transfer of female embryos only, or the use of donor sperm.
-
A specific genetic test from a blood sample is required. The primary difference between testing methods is based on the number of areas that are tested with anywhere from 6 to 24 areas being checked. There is limited value in testing more than 6-8 areas of the long-arm of the Y-chromosome. Hormone testing usually demonstrates an elevated FSH in the teens (normal being less than about 6) and modestly elevated LH; the testosterone is normal to low-normal. Testis size is usually diminished.
-
Y-MD testing is a very important test in the evaluation of males with infertility, most importantly in those with non-obstructive azospermia (as detailed above). MSP has declined to cover the costs. As of April 8, 2013 the costs have been passed along to patients. Your extended health may cover the costs.
PLEASE READ THESE INSTRUCTIONS CAREFULLY. FAILURE TO FOLLOW THESE INSTRUCTIONS MAY RESULTS IN A DELAY IN DIAGNOSIS AND TREATMENT
Make an appointment at LifeLabs to arrange for a blood draw. No special preparation required (i.e. you do not need to fast). LifeLabs may charge a fee of $30.
Make a payment for processing by contacting VGH cystogenetics.
Phone: 604-875-4068 Fax: 604-875-5210
Cost: approx. $225
Cash, cheque or credit card (VISA or MasterCard) to VGH cashier by phone or in person (Patient accounts Room 149 Centennial Building Vancouver General Hospital, 899 West 12th Avenue BC V5Z 1M9)
Notes:
Your specimen will not be processed without payment of the fee to VGH cytogenetics. Many patients incorrectly assume (or hope) that once the blood is drawn at LifeLabs that no further payments is necessary.
Test results take approximately 6 weeks to process. They are not available through the LifeLabs patient portal and will be sent to the ordering physician.
It is the patient’s responsibility to schedule their blood draw and to make payment for the testing.
This test result is essential in providing advice to patients who have few or no sperm in their ejaculate.
-
There are no known health repercussions from Y chromosome microdeletions except for infertility. It can be transmitted to male offspring. Depending on the chances that sperm will be found in the testis, a decision can be made if a microTESE is warranted. Note that some men with very mild (short and distal) Y-MD may have sperm in their ejaculate. Any sperm retrieved from males with Y-MD require IVF and ICSI to achieve a pregnancy.
Picture: Y-Chromosome: one of 46 chromosomes in the body. The long arm contains genes involved in sperm production. The AZFa, AZFb and AZFc are geographic areas that contain genes, but are not names of genes. These areas actually overlap. AZFa is at the 'proximal end' and AZFc at the 'distal end' of the long arm. The terminology is variable, but the basic concept is that there is missing genetic material from different areas of the Y-chromosome and in varying amounts which affects sperm production.
| Region(s) | Percent of Y-MD | Probability of Successful Sperm Retrieval |
|---|---|---|
| AZFc (b2/b4) | 65% | 50% |
| AZFb-c | 15% | <5% |
| AZFa | 10% | 15-75% (0% if complete) |
| AZFb | 10% | 60% |
| AZFa-b-c | 2% | 0% |
Table: Probability of finding sperm based on the extent of of the Y-chromosome micro deletion.
Congenital Absence of the Vas Deferens (CAVD/CBAVD) and Cystic Fibrosis
-
If the vas deferens is obstructed, sperm are blocked from passing into the ejaculate. Conception via intercourse is not possible. The most common cause of vasal obstruction is surgical - namely vasectomy. Some men, however, are born with obstruction of the vas deferens secondary to a genetic problem. There are 2 general types of genetic abnormalities which can occur:
CFTR gene related problem.
Non-CFTR gene related problem - rare accounting for less than 10% of men with an absent vas deferens.
Variants in the CFTR gene are responsible for cystic fibrosis and a group of other conditions that do not meet diagnostic criteria for CF, which include CBAVD.
Cystic fibrosis (CF) is an autosomal recessive condition which is common in individuals of Northern European ancestry. This means that abnormalities in both copies of the genes responsible for CF are required. Carriers (individuals with abnormalities in only one copy) do not have any clinical symptoms nor do they have any problems with fertility. Approximately 1 in 25 individuals of Northern European decent are carriers.
At conception, each sibling of an affected individual with CF and brothers of a male with CBAVD have a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffected and not a carrier. Carrier testing for at-risk relatives and prenatal testing for pregnancies at increased risk are possible if the CFTR pathogenic variants in the family are known.
This multisystem disorder affects mainly the respiratory and digestive tracts. CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene on chromosome 7. More than a thousand mutations have been identified in the CF gene. The most common mutation, F508del, accounts for approximately 70% of CF mutations in Northern Europeans. Direct mutation analysis for the most common mutations including F508del identifies approximately 90% of CF mutations in the Northern European population. The carrier frequency and DNA testing detection rate varies among other populations, but is generally less than that in the Northern European group. In all cases, unless there is a known mutation in a family, a negative result reduces, but does not eliminate the risk of an individual being a carrier for CF.
Congenital bilateral absence of the vas deferens (CBAVD) accounts for approximately 6% of cases of obstructive azoospermia and is responsible for 1-2% of cases of male infertility. Males with CBAVD due to CF mutations are not expected to exhibit any of the major symptoms of classic CF. CBAVD usually results from compound heterozygosity of a classic (severe, loss-of-function) CFTR pathogenic variant with a mild (retaining some function) CFTR pathogenic variant. However, some overlap exists between the CBAVD phenotype and a very mild CF phenotype, with a small percentage of individuals with CBAVD also reporting respiratory or pancreatic problems. There is a poly T tract within CFTR that is a genetic modifier of CF phenotype. This poly T tract can be associated with CFTR related disorders depending on its size. The three common variants of the poly T tract are 5T, 7T, and 9T. Both 7T and 9T are considered polymorphic variants and 5T is considered a variably penetrant variant.
The CFTR gene is responsible for secretions in the body. Problems with the CFTR gene result in cystic fibrosis (CF) - a condition in which secretions are thick. Thick secretions result in problems in the respiratory (lungs) and gastrointestinal tracts (pancreas, bowels), but can also affect other organs. Men with CF are born with normal reproductive tracts, but obstruction of the vas deferens and seminal vesicles by thick secretions eventually leads to scarring, atrophy and obstruction. On exam, the vas deferens is not palpable - or if it is, it is usually a thin, firm cord of tissue. Men with CF frequently have problems with infertility because sperm are blocked from making their way from the testis to the ejaculate. Men with CF-associated CBAVD present with CF, typically at a young age because of respiratory or other problems. Some men with a very mild form of CF present in adult life with infertility and none of the other symptoms which can be present with CF. In men presenting with this mild form of CFTR gene problem, there are no known long term health risks aside from reproductive ones. Occasionally, a patient with CBAVD may recall frequent episodes of bronchitis or sinusitis.
Other men have genetic abnormalities which do not affect the CFTR gene, but are missing the vas deferens. They may share some aspects of the genetic abnormalities seen with CF, but only have infertility. They do NOT have any of the other problems associated with CF, but there can be problems with development of the kidney on the same side that the vas deferens is absent.
-
The diagnosis of absence of the vas deferens is as simple as a physical examination and a semen analysis. The presence of a palpable vas deferens and intact epididymis effectively excludes the presence of CBAVD and genetic testing is not necessary. Men with CBAVD will have low-volume, acidic ejaculate without sperm. Hormone parameters are usually completely normal. Determining the underlying genetic cause, however, requires genetic testing on a blood sample. There are a very large number of genes which may result in CAVD or CF, but the majority are on chromosome 7. While genetic testing cannot currently assess all of the possible abnormalities, about 90% can be detected.
Most men with full blown CF have already had genetic testing. Men presenting later in life with CAVD require genetic testing to confirm the diagnosis. In non-CFTR related CAVD, an ultrasound of the kidneys is performed as it can be associated with absence of a kidney - this is not necessary if a CFTR-related defect is confirmed.
The most common genetic mutations associated with CBAVD are those of the F508 deletion and/or 5T mutation. Note that the 5T mutation is not seen in those patients will the full-blown cystic fibrosis (i.e. any clinical symptoms in addition to azospermia).
-
Virtually all men with CAVD (both those with CF and those without), have perfectly normal sperm production even though they are blocked from making their way to the ejaculate. Sperm can typically be retrieved either through a PESA/MESA or a TESE. Sperm obtained by these methods require IVF+ICSI.
It is recommended that the partners of men with CAVD be tested for CF carrier status. As mentioned previously, about 1 in 25 people of northern European descent will be carriers. If the partner is a carrier, there is an increased risk of the offspring having cystic fibrosis. If a man has CF and their partner is a carrier, then the risk that their offspring will have CF is 50%. If a man is only a carrier and their partner is a carrier, the risk that their offspring will have CF is 25%.
Consideration should be given to screening the family, especially male siblings, for carrier status.
DNA Fragmentation (DNAF) Testing
-
Sperm are basically transportation vessels for DNA. It is the content of the sperm, rather than the shape or how well the sperm moves, that primarily determines its reproductive ability. The assessment of male fertility has in large part relied upon the semen analysis - a microscopic evaluation which examines the ‘DNA container’ but does not evaluate the contents, the DNA itself. Routine semen analysis has significant obvious limitations and its use is largely confined to assessing the adequacy of the number of sperm present.
At this time, there is no method that can non-destructively assess the DNA of a single sperm. Therefore, any assessment is by necessity a general one of the population of sperm that are present. This is critical since it is the genetic content of a single sperm, mixed with that of a single egg, that results in the formation of an embryo.
There are 2 primary targets for assessment of DNA: (1) the chromosomes and genes themselves and (2) how the physical strands of DNA which contain the information is packaged. Karyotypes analysis and Y chromosome deletions seek to assess the first target whereas DNA fragmentation seeks to assess the packaging.
DNA fragmentation is an attempt to determine how well the DNA has been packaged within a sperm and by extension may provide insight in to damage that might have occurred to the DNA itself.
Sperm in DNA is different from that in other cells - it is specially packaged for travel. One might intuitively think that if the DNA packaging is ‘broken’ that the information within the package might be compromised and that there would be a reduced chance of reproductive success. This has been shown to be the case. DNA fragmentation is associated with lower fertilization rates, pregnancy rates and live birth rates and with a higher rate of pregnancy loss.
This package may be damaged by multiple potential causes. Known and proposed causes of DNA fragmentation include chemical agents (cigarettes, chemotherapy, pesticides), biological agents (increasing male age, obesity, diabetes, sexually transmitted diseases) and physical agents (radiation, heat).
Despite these correlations, DNA fragmentation testing remains controversial. There are several reasons:
DNAF testing in part answers a question that we already know the answer to. We already know that the patient is infertile - this is why the test is done - so a positive test simply confirms what we already know.
There is no consensus on the best testing procedures, clinical reference values or management of patients with increased DNAF. It is unclear if there is any benefit to reducing DNAF or retrieving sperm that are less likely to have DNAF (e.g. testicular sperm).
The large randomized trials that would answer these questions have not been performed.
As a consequence, almost all professional bodies and authors of clinical guidelines do not yet support the routine testing for sperm DNA fragmentation.
References
Gat et al. Sperm DNA fragmentation index does not correlate with blastocyst euploidy rate in a donor cycles. Gynecological Endocrinology 2017.
Esteves et al. Reproductive outcomes of testicular bruises ejaculated sperm for intracytoplasmic sperm injection a month and with high levels of DNA fragmentation in semen: Systematic review and meta-analysis. Fertility Sterility 2017.
Halpern and Schlegel. Should a couple with failed in vitro fertilization/intracytoplasmic sperm injection and increased sperm DNA fragmentation use testicular sperm for next cycle? European Urology Focus 2018.
Metha et al. Use of testicular sperm in non-azoospermic males. Fertility Sterility 2018. ***
Pacey. Is sperm DNA fragmentation a useful test that identifies a treatable cause of male infertility? Best Practice and Research Clinical Obstetrics and Gyneaecology 2018. ***
Agarwal et al. Sperm DNA fragmentation: A new guideline for clinicians. Male Reproductive Health and infertility 2020.
Jarvi. High sperm DNA damage. Does testicular sperm make sense? Urologic Clinics of North America 2020. doi.org/10.1016/j.ucl/2019.12.009 ***
-
DNAF testing may be performed on sperm obtained by any method. While it is usually performed on ejaculated sperm, surgically retrieved sperm may also be tested.
Testing is not covered by the Medical Services Plan and the cost is generally in the range of $750. Check with your fertility centre for information.
There are several methods to assess how much fragmentation is present.
SCSA: sperm chromatin structure assay.
SCD: sperm chromatin dispersion test.
TUNEL: terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick end labelling assay.
Comet: single cell gel electrophoresis assay.
A test is just a tool that can be used to help make a diagnosis and/or make better decisions. Understanding how the tool works, when to use it and the limitations of the tool are critical.
Of the available assays, the TUNEL and Comet are more accurate at identifying DNA fragmentation when it is present (i.e. more sensitive). SCSA and SCD are less accurate at detecting DNA fragmentation (i.e. less sensitive). Despite being more accurate, both TUNEL and Comet are relatively poor tests when assessed using standard measures of test performance. For example, the area under the curve (AUC) using standard ROC methods shows that TUNEL and Comet have an AUC value of between 0.71-0.73. For reference, a very good test will have an AUC in the range of 0.9 and a useless test will have an AUC of 0.5. TUNEL and Comet have middling sensitivity. Specificity, or the ability of a test to correctly identify people without the disease, is also an issue (see more below). doi:10.1371/journal.pone.0165125
The lab reports frequently calculate the ‘DNAF Index’ which is the proportion of cells that have damaged DNA. There are no accepted thresholds that define what might be considered normal or insignificant and abnormal or significant DNA fragmentation. More DNAF is worse but how much worse at any particular level of DNAF and in any particular scenario is an unanswered question.
When assessing the result of a DNAF test, the predictive value of the result will vary depend on the prevalence of the disease in the population of interest (i.e. it will vary for each clinical scenario) and after controlling for each of the potential confounding variables (i.e. all other male or female factor that may be playing a role). One should appreciate that this becomes very, very messy since unless the test has been validated in the scenario in which it is being used, the test may in fact be useless. For example, the implications of a DNAF test might be different depending on the sperm concentration, the type of conception (intercourse, insemination, IVF, IVF-ICSI), any number of female factors (e.g. age), prior pregnancy history, etc. The number of permutations in common clinical practice is huge and this severely limits applicability of this test. Therefore, most of the focus has been on couples who have the most challenging scenarios: repeated failure of eggs to fertilize, repeated early pregnancy loss, etc. One should be cautious in using a tool in scenarios where it has not been validated.
Lastly, there is data that implies that false positive results for the DNAF testing may be more common than true positives (doing:10.1016/j.fernstert.2007..04.055). In plain English, this means that the sperm of a man identified as having high DNAF may in fact not have any fragmentation - that the test wrongly identifies a problem when none exists.
One should be cautious about drawing any definitive conclusions from DNAF testing and if one decides to obtain such testing to avoid ascribing more precision to the result than is deserved.
The reports from the companies providing results are commonly presented with a facade of accuracy and precision that belies all of the uncertainty and lack of precision that are inherent in the results.
For those interested in reading more on their own, I recommend 2 papers: Collins et al. cover many of the principles and issues of using DNAF testing clinically with the discussion of predictive values on p. 830 being of particular importance (doi:10.1016/j.fertnstert.2007.04.055). Pacey updates the discussion with more recent data (doi:10.1016/j.bpbhun.2018.09.003).
-
The management of DNA fragmentation remains controversial. There are no proven ways to reduce fragmentation with the exception of the most extreme scenarios (e.g. chemotherapy or radiation - in which removal of the insult allows for self-correction).
The counsel that each couple receives will often need to be individualized to their specific circumstances and couples must be aware that evidence-based recommendations are challenging or impossible given the complex nature of infertility the absence of large scale trials. At most, DNAF testing will play a small role and should never be relied upon in isolation for clinical decision making.
General Measures
One might hypothesize that the use of antioxidants or avoidance of oxidative damage would be helpful. The data are mixed. Having said that, most of the recommendations are good for one’s general health and carry little if any risk.
Minimize or eliminate exposure to cigarettes and marijuana.
Exercise to reduce obesity.
Eat a health balanced diet that is high in fruits and vegetables and low in fat.
Supplements are likely of limited value but if one must, consider vitamins C, E, B12, L-carnitine, coenzyme Q10, folic acid. The studies showing benefit have usually be performed by the companies selling the product.
Specific Interventions
There are 2 interventions that have been proposed to correct or circumvent the problem.
The first is varicocele correction. Oxidative damage by heat or other varicocele-related injury may contribute to infertility. In most cases, there will be other features to recommend varicocele correction (e.g. oligospermia, large varicocele and infertility).
The second is the use of testicular sperm in conjunction with IVF-ICSI. The hypothesis is that the degree of oxidative damage to sperm is proportional to the time the sperm has spent in the reproductive tract. Sperm in the testis, by definition, have spent the least amount of time in the reproductive tract and have less oxidative damage. This has been proven, however, the benefits of utilizing testicular sperm remain controversial.
Ejac-ICSI is the use of ejaculated sperm for intracytoplasmic sperm ejaculation whereas Testi-ICSI is the use of surgically retrieved sperm from the testicle for ICSI. There is some data to suggest both an improved fertilization rate and live birth rate when Testi-ICSI is performed. Benefit with this approach was more likely in couples in which the man had high DNAF, normal sperm counts and when the couple had already failed ICSI before where the live birth rate was roughly 50% with Testi-ICSI and roughly 25% with Ejac-ICSI. Other studies have not shown any difference (doi/10.1016/j.fertnstert.2017.06.018). The decision to proceed with testiscular sperm extraction (TESE) in this setting remains controversial (doi/10.1016/j.fertnstert.2018.04.029).
While TESE is not a major undertaking, couples should consider the potential complications and risks in deciding whether to proceed. Individualized counselling is mandatory.
Other Types of Genetic Abnormalities
Because there are many chromosomes and genes involved in sperm production, there are a very large number of potential genetic causes for infertility in a man. We are not currently able to test for all causes, though this will likely be possible in the future. In addition to the 3 most common types of genetic abnormalities responsible for infertility, there are many less common types. The main importance of identifying these conditions is that pre-implantation genetic testing may be indicated to reduce the risk of transmission to offspring. In addition, some types of genetic abnormalities can preclude the presence of spermatogenesis and therefore save a man the cost and complications of undergoing a futile attempt at sperm retrieval.
Other types of genetic abnormalities include:
Aneuploidy: this is an issue with the number of chromosomes. The normal complement in a male is 46 chromosomes, 2 of which are sex chromosomes (X and Y). Examples: Klinefelter's is an example of aneuploidy involving an extra X chromosome - 47 XXY. Down Syndrome have an extra copy of chromosome 21.
Deletions: portion of a chromosome is missing.
Inversions: portion of a chromosome is broken off, turned upside down and reattached.
Translocations: when a segment of one chromosome is transferred to another chromosome. Sometimes information from one chromosome is 'swapped' with information for another. As long as all the information is there, the individual does not usually have a problem. If offspring can be conceived, they may have major congenital defects because they do not have a full 'instruction set'.
Genetic Counseling
The approach to managing males with genetic problems causing infertility, the entire family including the partner and the potential offspring must be considered. Implications regarding transmission of genes to the offspring and the long-term implications is important. The ultimate goal is a healthy, happy family. Genetic counseling is recommended for all individuals. The fertility labs may offer genetic counseling as does the Provincial Medical Genetics Program. Unfortunately, it can take a long time to obtain an appointment at BC Women's Hospital.